Videos & Webinars
Watch videos featuring supply chain experts
The U.S. pharmaceutical industry is one of the largest and most influential sectors in the global economy, playing a critical role in healthcare by producing innovative drugs, vaccines, and medical treatments. Home to some of the world’s leading pharmaceutical companies, the U.S. has long been at the forefront of pharmaceutical research, development, and exports. This industry not only supports millions of jobs domestically but also addresses worldwide healthcare needs by supplying high-quality pharmaceutical products to countries around the globe.
Over the past few years, the U.S. pharmaceutical import landscape has been evolving due to a combination of declining imports from traditional suppliers like China, India, and European countries. Understanding these shifts requires an analysis of various factors, including geopolitical tensions, trade agreements, regulatory challenges, and supply chain disruptions, as well as opportunities created by new entrants in the market.
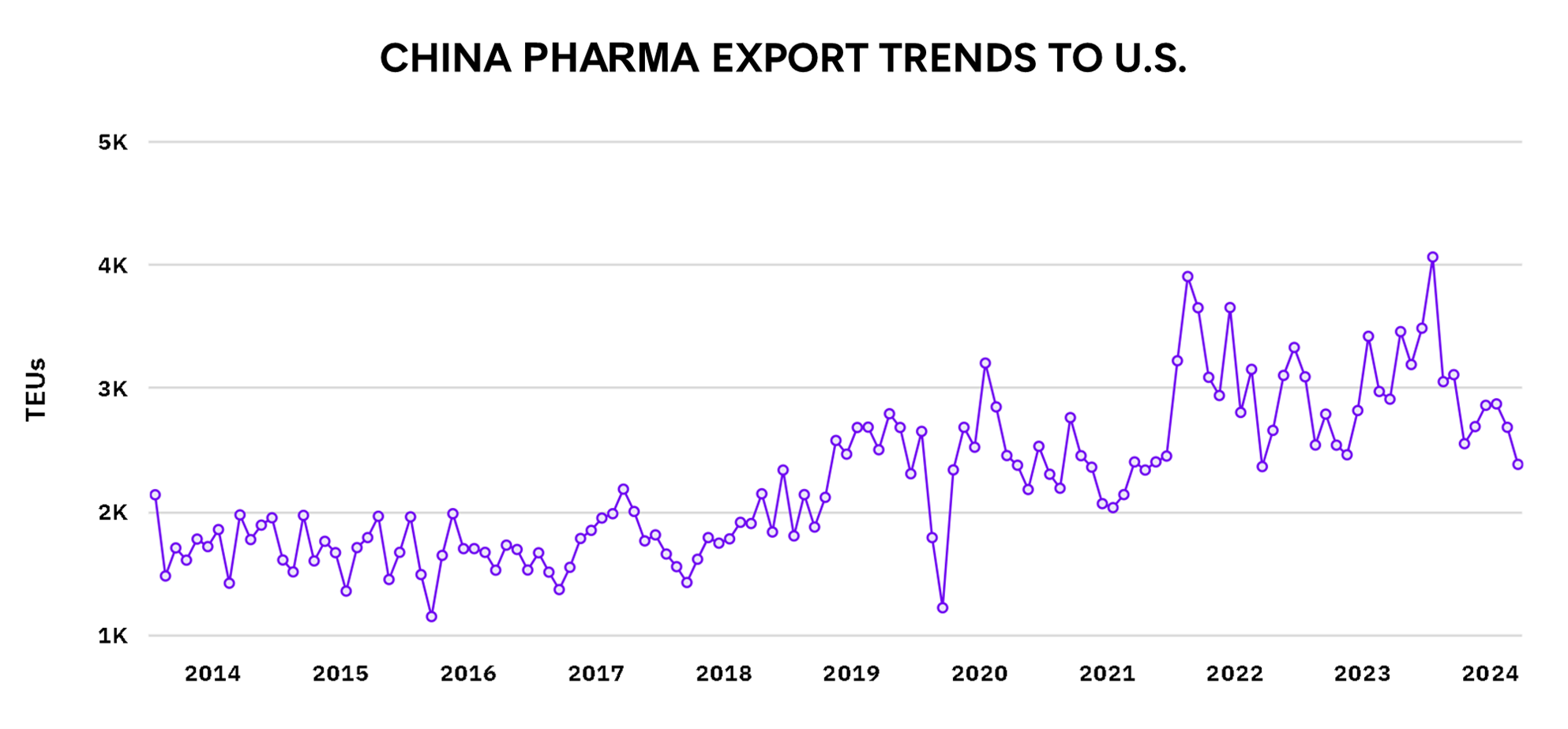
From 2019-2022, China was a significant exporter of pharmaceutical goods to the U.S., but we see a reduction in this volume in the 2022-2024 period. Several key factors explain this:
U.S.-China Trade War and Tariffs: Starting in 2018, the trade war between the U.S. and China escalated with each country imposing tariffs on various goods. Pharmaceuticals weren’t heavily impacted initially, but the overall tense trade environment created uncertainties. For some Chinese pharmaceutical products, higher tariffs were applied, which made these goods more expensive for U.S. buyers. This led U.S. companies to look for alternative suppliers who could provide lower-cost products without the added tax burden.
Supply Chain Disruptions from COVID-19: The pandemic severely affected manufacturing and shipping worldwide, but especially in China due to strict lockdowns and travel restrictions. Even though pharmaceuticals were deemed essential and continued production, logistical challenges—such as port closures, shipping delays, and a shortage of cargo capacity—slowed down exports from China. These disruptions caused U.S. companies to explore other countries like India and European suppliers for more reliable pharmaceutical imports.
U.S. Diversification Efforts: The U.S. has been actively working to reduce its dependency on China for critical goods, including pharmaceuticals. This has been a strategic move to prevent over-reliance on a single country for essential medicines. The U.S. government has encouraged domestic production and sought to establish partnerships with other countries to increase supply chain resilience. This policy shift led to a decline in pharmaceutical imports from China as U.S. companies sourced their products elsewhere.
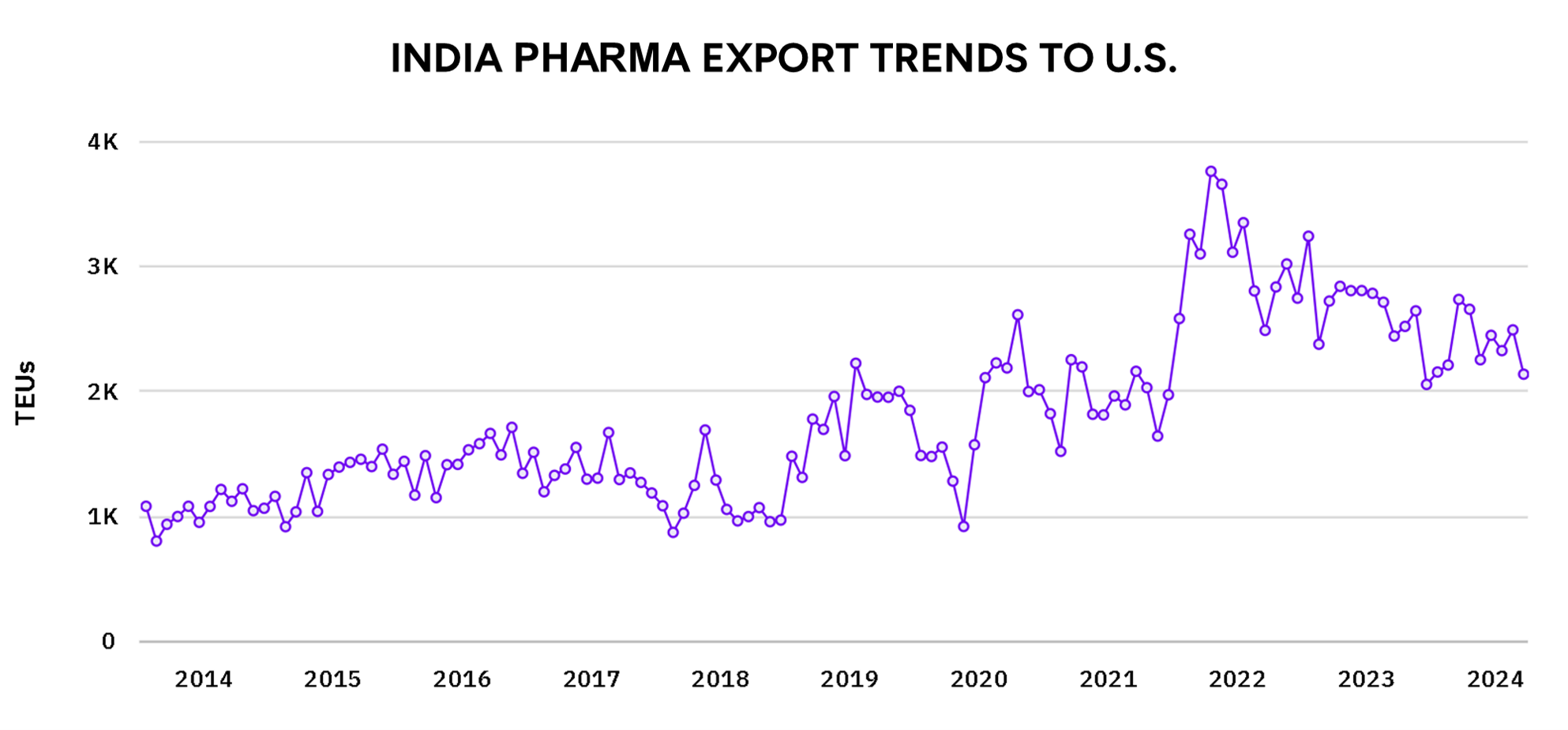
India is the world’s largest provider of generic medicines, but the data shows a slower growth in pharmaceutical exports to the U.S. between 2022 and 2024. Here are the main reasons:
Pressure on Generic Drug Prices: India exports a large volume of low-cost generic drugs to the U.S. However, the U.S. healthcare system has been trying to control costs by pushing down the prices it pays for these drugs. The U.S. government and insurance companies have implemented pricing controls and promoted local manufacturing to reduce their dependency on imports. This price pressure affects Indian exporters because they operate on thin margins and cannot always lower their prices further. As a result, while India’s export volumes remain stable, the growth rate has slowed.
Regulatory Scrutiny from the U.S. FDA: The U.S. Food and Drug Administration (FDA) plays a crucial role in approving drugs for import. Over the years, many Indian pharmaceutical companies have faced challenges with quality control and compliance issues in their manufacturing plants. The FDA has frequently imposed bans on specific Indian factories for failing to meet stringent quality standards, which limits the ability of Indian companies to expand their exports to the U.S. Ensuring compliance with FDA regulations has become more costly and time-consuming for Indian firms.
Focus on Domestic and Global Vaccine Production: During the COVID-19 pandemic, India shifted much of its focus to producing vaccines, including the AstraZeneca vaccine, which India manufactured in large volumes under the name Covishield. This global demand for vaccines redirected India’s pharmaceutical production capacity away from other drugs, including those destined for the U.S. market. Even though vaccine production was a priority, it reduced India’s ability to increase its exports of generic medicines to the U.S.
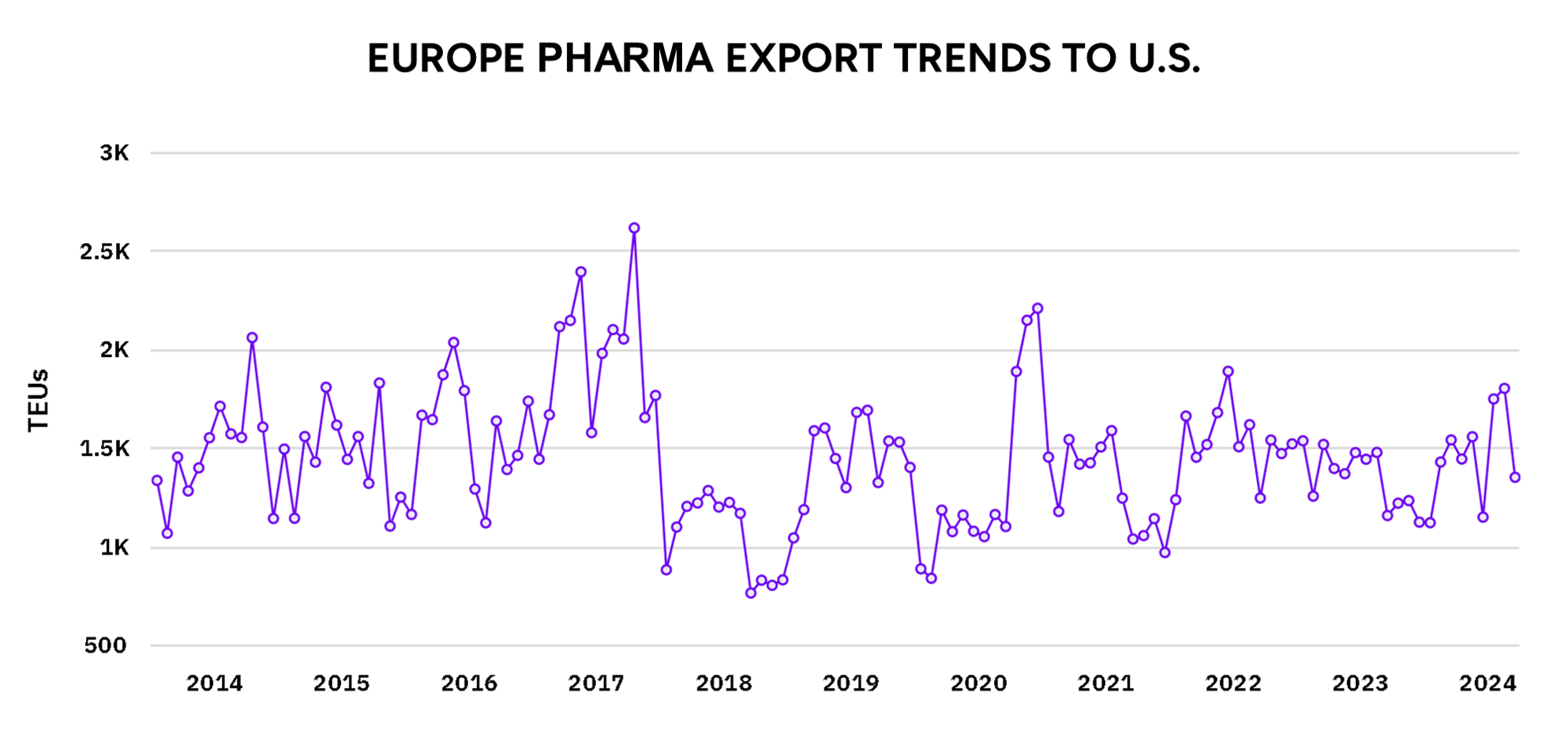
Exports from major European countries like Germany, France, and Switzerland have also seen a decline. These countries are known for producing high-quality, innovative pharmaceutical products, but several factors are at play:
Higher Production Costs in Europe: European pharmaceutical companies often produce more complex, innovative drugs, which are typically more expensive to manufacture compared to the generics coming from countries like India. In recent years, the European Union (EU) has implemented stricter environmental and pharmaceutical regulations, such as guidelines for carbon emissions and tighter controls on chemical waste from factories. These regulations have increased the cost of manufacturing, making European drugs less competitive in the U.S. market.
Shifts in Investment Priorities: During the pandemic, European governments and companies began investing more heavily in the domestic production of pharmaceuticals to ensure self-sufficiency. The focus was on building resilient supply chains that could withstand future crises like COVID-19. These investments redirected resources that might have otherwise been used to expand exports to markets like the U.S.
Increased Domestic Demand in Europe: The pandemic caused a surge in domestic demand for pharmaceuticals across Europe. European countries prioritized using their production capacity to meet the healthcare needs of their populations, especially for critical medicines and vaccines. This left less capacity for exports to the U.S., leading to a reduction in volumes.
Impact of Brexit on the U.K. Pharmaceutical Sector: The United Kingdom was a significant player in pharmaceutical exports to the U.S., but Brexit has created several complications. Since leaving the European Union, U.K. pharmaceutical companies have faced additional bureaucratic hurdles, such as new documentation requirements and customs checks. Additionally, the regulatory divergence between the U.K. and the EU has made it more difficult for U.K. companies to export their products, as they need to comply with separate regulations for the EU and other countries like the U.S. These factors have contributed to a slowdown in U.K. pharmaceutical exports to the U.S.
In addition to the decline or stagnant growth in U.S. pharmaceutical imports from traditional players like China, India, and Europe, the graphs you provided also indicate the emergence of new market players that are beginning to influence the U.S. pharmaceutical import landscape. Here's a more detailed analysis of these emerging markets and what may be driving their growth.
The U.S. pharmaceutical export landscape has undergone significant changes from 2022 to 2024, influenced by several global and domestic factors. Imports from traditional suppliers such as China, India, and European nations have declined or experienced stagnation due to a combination of trade tensions, regulatory challenges, and shifting domestic priorities. China's exports were affected by the U.S.-China trade war and supply chain disruptions, while India's pharmaceutical growth slowed due to price pressures and stricter FDA regulations. Europe, faced with rising production costs, increased domestic demand, and Brexit complications, also saw a reduction in exports to the U.S.